Erosive tooth wear is a common oral condition that increases in severity with age. It is most commonly caused by acids in our food and drinks and involves the chemical and physical etching away of hard dental tissue. If left unmanaged, it can result in complete degradation of enamel and exposure of dentine, leaving teeth smaller with a dull yellow appearance and causing tooth sensitivity and pain. A research team at King’s College London has been working on ways to improve the understanding of the tooth wear process itself and investigating innovative techniques for clinicians to monitor and treat it.

Above. Dr Polyvios Charalambous, member of the research team.
Assessing a new tool
“Currently, there is a pressing requirement for the development of quantitative in vivo methods to measure tooth wear directly on patients” explains Dr Polyvios Charalambous. “Conventional methods for clinically monitoring tooth wear such as tooth wear indices, dental casts and clinical photography all rely on subjective qualitative visual feedback. Advancements in 3D imaging and digital surface measurement have allowed quantitative methods to be developed which involve taking dental impressions of patients at different points in time, pouring them into dental stone to construct dental casts and scanning these with a 3D scanner to quantify surface changes using dedicated software. These workflows are complicated and cumbersome, consisting of multiple steps and requiring highly expensive equipment and skilled operators, limiting them to an academic setting.”
“Intra-oral scanners (IOS) are handheld clinical devices that can capture the 3D geometry of teeth inside a patient’s mouth with the aim of replacing the need for conventional dental impressions” Polyvios continues. “They are becoming more common in the clinical setting and whilst primarily designed for digital production of CAD/CAM restorations, can provide the required digital surface models. There is therefore a potential for in vivo quantification of surface changes on teeth and dental materials using surface metrology principles.”
“Investigating the performance of intra-oral scanners in quantifying tooth wear on naturally curved tooth surfaces is ideal as it is more clinically relevant and is the focus of our current and future research. Our first goal was to determine the intra-oral scanner’s limitations by using a controlled and standardized wear model, thus reducing measurement errors commonly seen in surface imaging algorithms. Flat, polished samples allow vertical enamel loss measurements in the form of step heights by comparing the flat reference datums on either side of the wear lesion and the lesion itself” he explains.
“Our research team aimed to determine the measurement limits of a commonly used intra-oral scanner, compared to a highly accurate 3D profilometer as the gold standard for measuring wear changes on polished human enamel (depths of 21.8–269.0μm) as well as changes on surfaces with increasing roughness.” To the authors’ knowledge, this is the first study in which datasets from intra-oral scanners were analyzed with Mountains® software.
“Our study reports new findings combining optimized imaging workflows with intra-oral scanning which revealed for the first time that intra-oral scanning can only detect Sq surface roughness of silicon carbide particle sizes above 68.0μm. This highlights limitations in measuring short-wavelength surface features compared to profilometry. In terms of step height detection, an automated lesion localization algorithm revealed the measurement threshold of the intra-oral scanner to be 44.02μm, showing for the first time that enamel lesions can be reliably quantified using automated detection at the sub-50μm level. This is comparable to the level of annual wear of human enamel, estimated to be around 38μm (Lambrechts et al. 1989). This finding shows promise for intra-oral scanners as diagnostic and research tools for quantifying tooth or material wear; however, further technological advancements are still required.”
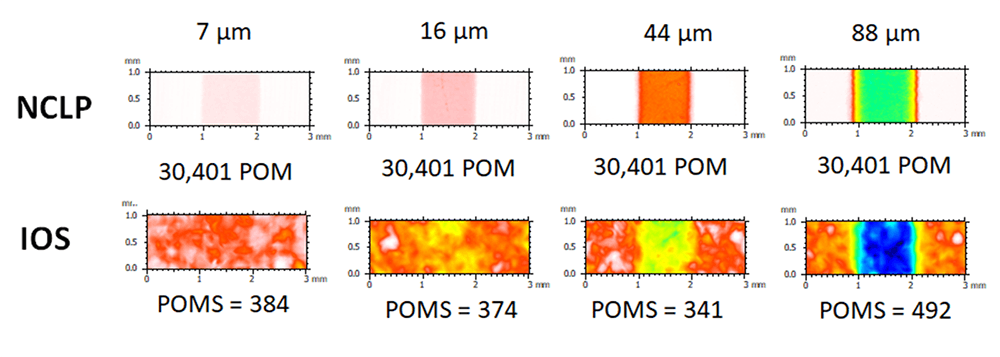
Above. Measurements (µm) of lesion depth using a non-contact laser profilometer (NCLP) and an Intra-oral scanner (IOS).
Automating analysis
“The unique functionalities of Mountains® software helped us develop an automated lesion detection and analysis workflow. To investigate the measurement limitations of IOS, measurements of Sq roughness, 3D surface step heights (ISO 5436-1) and XY wear lesion areas were compared with those of the non-contact laser profilometer (NCLP), corroborated by Gaussian skewness (Ssk) and kurtosis (Sku) analysis.
Firstly, the 3D step height (in μm) of the central lesion was calculated using a predefined selection of the polished enamel reference areas compared to the lesion, based on ISO 5436-1. Using Mountains®, these predefined areas were programmed to automatically have the same size and location on each NCLP and IOS digital surface.”
Example of an IOS digital surface:
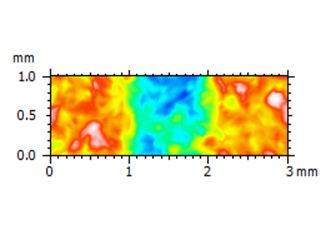
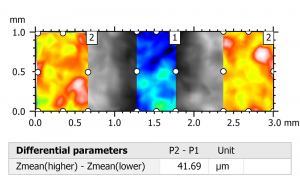
Above left. Pseudo-color view of the automated lesion surface using an intraoral scanner. Above right. Step height analysis on the pre-defined surface.
Example of an NCLP digital surface:
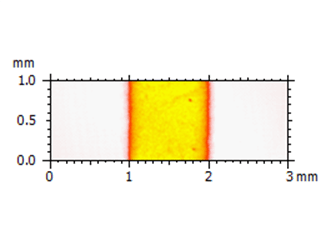
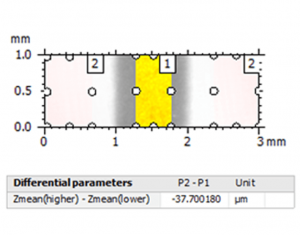
Above left. Pseudo-color view of the same surface using a non-contact laser profilometer (NCLP). Above right. Step height analysis of the surface.
“Secondly, an automated lesion localization algorithm was used to determine the measurement threshold of the IOS by localizing and measuring the XY lesion area (in mm2) on each scan. We used the Particle analysis feature of the software, which detected surface points of measurement (POM) with Z-amplitudes below the mean plane of the surface heights according to the histogram of the surface heights distribution, on each 3×1mm dataset. The Z-heights of surface points inside a given lesion would be below the mean plane of surface heights in an otherwise planar surface, resulting in lesion localization. The surface area (in mm2) of these points was calculated for each IOS scan and compared to the NCLP.”
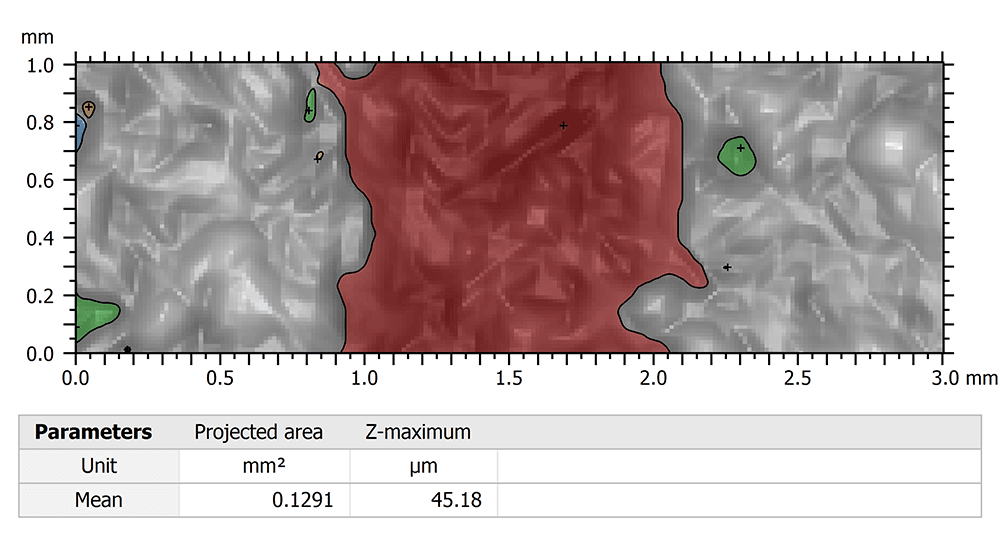
Above. Particle analysis of lesion using an IOS scan.
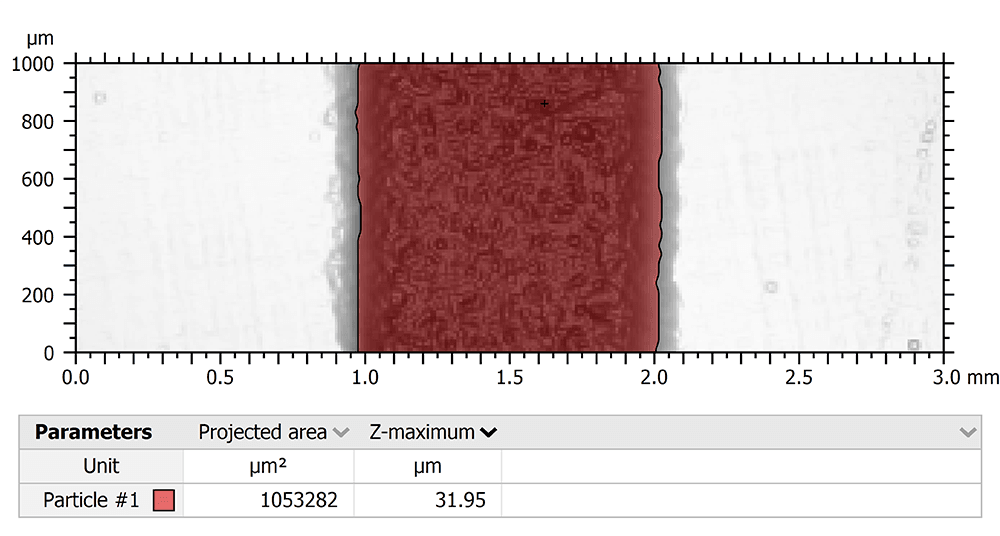
Above. Particle analysis of lesion using an NCLP scan.
“Finally, the Gaussian surface height distribution was analyzed on each 3×1mm dataset using surface skewness (Ssk) and kurtosis (Sku) parameters. The surface distribution curve of a nominally flat surface superimposed with a random roughness has a Gaussian distribution symmetrical about the height of the nominal plane which is quantified by Ssk equal to zero and Sku equal to three. In contrast a surface with a central lesion will display negative skewness and kurtosis less than three.”
Instruments & software used
True Definition intra-oral scanner by Midmark, TaiCaan Technologies XYRIS 2000 CL profilometer + MountainsMap® software (Step height study & particle analysis).
The measurement threshold and limitations of an intra-oral scanner on polished human enamel. P. Charalambous, S. O’Toole, T. Bull, D. Bartlett, R. Austin. In: Dental Materials, available online. https://doi.org/10.1016/j.dental.2021.01.006
Acknowledgements:
This research was co-funded by the Medical Research Council and GlaxoSmithKline Consumer Healthcare Oral Health Research & Development.